Abstract
Background: The feasibility/safety of outpatient (outpt) HMA + Ven administration and use of Ven ramp-up to target dose during cycle 1 (C1) for patients (pts) with newly-diagnosed AML is limited to small, single-center analyses of heterogeneous populations. Furthermore, appropriate pt selection for varying approaches is not well-described. Understanding these issues is crucial to efficient and cost-effective care delivery, especially considering the unique challenges older pts face.
In this multi-center analysis, we examined the feasibility and safety of frontline HMA + Ven administration in the outpt setting as well as omission of Ven ramp-up during C1.
Methods: We conducted a multi-center, retrospective study through the Consortium on Myeloid Malignancies and Neoplastic Diseases (COMMAND), a collaboration involving 10 academic institutions. A total of 272 pts with AML treated with frontline HMA + Ven were evaluated; pts presenting in spontaneous tumor lysis syndrome (TLS) or with incomplete data were excluded (n=24). We described the baseline characteristics of the overall study population. Continuous variables were summarized as a median with interquartile range (IQR), and categorical variables were reported as a frequency (percentage). Fisher's exact test was used for nominal data and Wilcoxon rank-sum testing was used for continuous variables
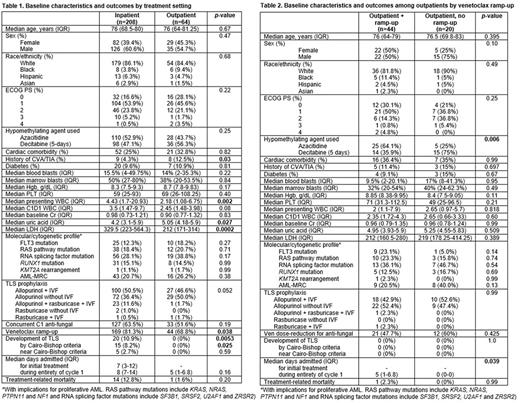
Results: A total of 64 pts (23.5%) initiated HMA + Ven as outpts. Median age was 76 (IQR: 64-81.25) years, and the majority were male (n=35, 54.7%), white (n=54, 84.4%) and had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-1 (n=42, 73.7%). Cardiac comorbidities, prior stroke and diabetes were noted in 32.8%, 12.5% and 10.9% of outpts, respectively. Median total white blood cell count (WBC), marrow and peripheral blasts at presentation were 2.18 (IQR: 1.08-6.75), 38% (IQR: 20-53.5%) and 14% (IQR: 2-35.3%), respectively. Pre-treatment WBC, serum creatinine, uric acid and LDH were noted to be 2.45 (IQR: 1.48-3.98), 0.90 (IQR: 0.77-1.32), 5.05 (IQR: 4.18-5.9) and 212 (IQR: 171-314), respectively. All outpts had a pre-treatment WBC <20, uric acid <10 and creatinine <2; 8 pts (12.5%) had an LDH >500. Baseline characteristics are summarized in Table 1.
When compared with pts receiving C1 of HMA + Ven as an inpt (n=208), outpts were found to have a lower presenting WBC (p=0.002), trend towards lower pre-treatment WBC (p=0.08) and a lower LDH (p=0.0002); pre-treatment uric acid was higher among outpts (p=0.027)(Table 1). There were no differences in disease with mutations with an influence on proliferative AML. Most outpts received allopurinol TLS prophylaxis (ppx); inpts more frequently had rasburicase ppx (13.1% vs. 3.4%, p=0.052).
Most outpts received azacitidine (n=36, 56.3%) vs decitabine (5-day schedule)(n=28, 43.7%) with a similar distribution among inpts (p=0.25). Approximately half of outpts (51.6%) received anti-fungal ppx and required Ven dose reduction during C1. TLS was not observed in any outpts, compared with 10% in inpatients (p=0.0053) including 8% by Cairo-Bishop criteria (p=0.025). There was no difference in the median number of days admitted during the entirety of C1 between inpts and outpts (8 vs 5, p=0.16); 11% of outpts were eventually admitted during C1. Although treatment-related mortality (TRM = up to 90 days from start of C1) was higher among inpts, this was not statistically significant (12.8% vs 1.6%, p=0.20)(Table 1).
Among outpts, approximately 1/3 had no Ven ramp-up (n=20, 31.3%). When compared with outpts with Ven ramp-up (n=44, 68.7%), there were no differences observed in age, ECOG PS, comorbidities, molecular characteristics, presenting or pre-C1 WBC, marrow/blood blasts, serum creatinine, uric acid, LDH, TLS ppx patterns or Ven dose-reduction for antifungal ppx (Table 2). More outpts without Ven ramp-up received decitabine (75% vs 35.9%, p=0.006). Median number of days admitted was higher among outpts with Ven ramp-up (5 vs 0, p=0.039) with one TRM attributed to infection in this group (p=0.99)
Conclusions: Our study demonstrates that many pts with newly-diagnosed AML can safely receive C1 of HMA + Ven as an outpt, particularly those with a pre-treatment WBC <20, uric acid <10 and creatinine <2. We observed no TLS among outpts, including those without Ven ramp-up, which may be safely omitted in select pts. Prospective studies are needed to confirm these findings.
Disclosures
Shallis:Bristol Myers Squibb and Gilead Sciences, Inc: Honoraria; Gilead Sciences, Inc.: Honoraria. Winer:Abbvie: Consultancy; Takeda Pharmaceuticals: Consultancy; Novartis, Jazz Pharmaceuticals, Pfizer, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy. Im:Incyte: Research Funding; CTI Biopharma: Consultancy; Abbvie: Consultancy. Abaza:ALX Oncology: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Litzow:Abbvie: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Syndax: Research Funding; Jazz: Consultancy; Actinium: Research Funding; Pluristem: Research Funding; Biosight: Other: Data Monitoring Board. Podoltsev:Constellation Pharmaceuticals: Honoraria; AbbVie: Honoraria; Cogent Biosciences: Other: Independent Data Review Committee ; Pfizer: Honoraria; Agios Pharmaceuticals: Honoraria; Blueprint Medicines: Honoraria; Incyte: Honoraria; CTI BioPharma: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; PharmaEssentia: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal